Publications
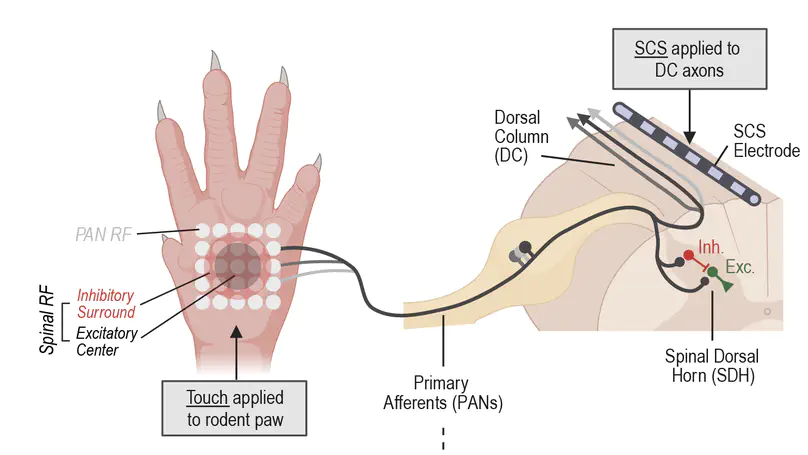
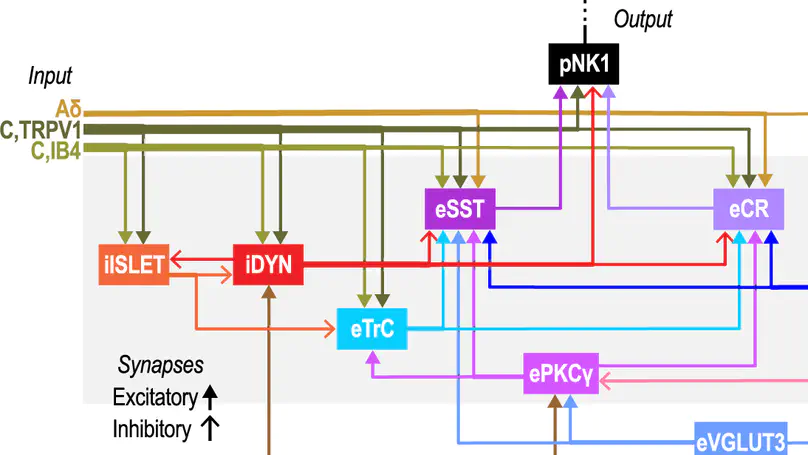
Touch is mistakenly perceived as painful when inhibition in the spinal dorsal horn (SDH) is weakened. Disinhibition un-gates a polysynaptic spinal circuit but why mechanical allodynia is predominantly evoked by certain stimuli, like dynamic brushing, remains unclear. To answer this, we incorporated receptive fields (RFs) into a computational model of the SDH to study the processing of stimuli with different spatiotemporal features. Broad stimuli normally suppress output spiking by engaging inhibition from the RF surround, but the efficacy of inhibition depends on the input’s temporal pattern. This is critical since excitatory and inhibitory spinal neurons are preferentially sensitive to synchronous and asynchronous input, respectively. Furthermore, spikes driven by synchronous input are resistant to feedforward inhibition. The combined results explain why broad dynamic touch (e.g. brush or vibration) evokes more allodynia than punctate static touch. Our results also show that asynchronous and spatially disordered input evoked by kilohertz-frequency spinal cord stimulation preferentially activates inhibitory neurons, thus explaining its anti-allodynic effects.
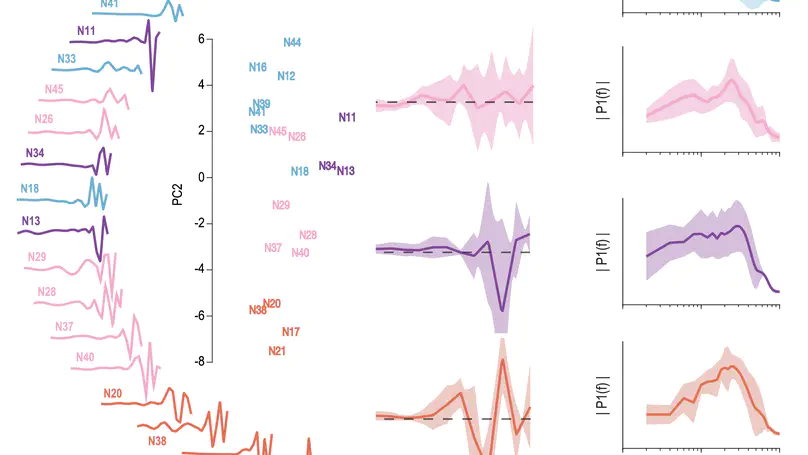
Our sense of touch starts with activation of nerve fibres in the skin. Although response properties of various fibre types are well-established in other species (e.g. primates), quantitative characterization in rats and mice is limited. To fill this gap, we performed a comprehensive electrophysiological investigation into the coding properties of tactile fibres in rodent non-hairy skin and then simulated these fibres to explain differences in their responses. We show that rodent tactile fibres resemble those from other species, but that their heterogeneity at the population level may differ, with potentially important implications for encoding of touch. Simulations reveal intrinsic mechanisms that support this heterogeneity and provide a useful tool to explore somatosensation in rodents.
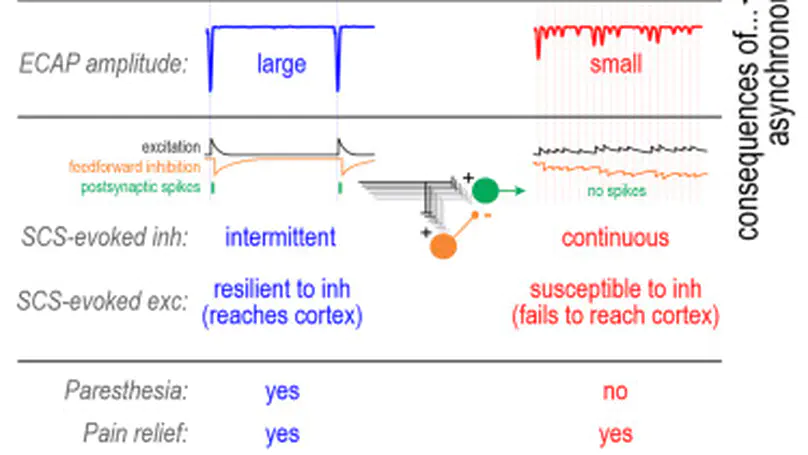
Electrically activating mechanoreceptive afferents inhibits pain. However, paresthesia evoked by spinal cord stimulation (SCS) at 40–60 Hz becomes uncomfortable at high pulse amplitudes, limiting SCS “dosage.” Kilohertz-frequency SCS produces analgesia without paresthesia and is thought, therefore, not to activate afferent axons. We show that paresthesia is absent not because axons do not spike but because they spike asynchronously. In a pain patient, selectively increasing SCS frequency abolished paresthesia and epidurally recorded evoked compound action potentials (ECAPs). Dependence of ECAP amplitude on SCS frequency was reproduced in pigs, rats, and computer simulations and is explained by overdrive desynchronization, spikes desychronize when axons are stimulated faster than their refractory period. Unlike synchronous spikes, asynchronous spikes fail to produce paresthesia because their transmission to somatosensory cortex is blocked by feedforward inhibition. Our results demonstrate how stimulation frequency impacts synchrony based on axon properties and how synchrony impacts sensation based on circuit properties.